The Guardianship and Administration Act 1990 (The Act) Part 9E provides the authorisation and safeguards for consent to be given for a person with a decision-making disability to participate in approved medical research. This research must have been approved by a Human Research Ethics Committee that complies with the National Statement on Ethical Conduct in Human Research issued under the National Health and Medical Research Council Act 1992 (Cth).
What is medical research?
The term medical research means research conducted with or about individuals, or their data or tissue, in the field of medicine or health; and includes an activity undertaken for the purpose of that research.
Medical research does not include research about individuals, or their data or tissue that only analyses such data and does not result in the disclosure or publication of personal information.
The Research candidate
A person being considered for, or provided with, treatments in approved medical research is called the ‘research candidate’. The Act specifies what must be considered before approval is given for a person to be a research candidate.
The Research decision-maker
A person who has the authority to consent on behalf of a person with a decision-making disability to participate in medical research is called the ‘research decision-maker’. The Act specifies when a person can be a research decision-maker: see the ‘Process for obtaining a research decision’ [LINK] below for further details.
The research decision-maker, on advice from an independent medical practitioner, provides or refuses consent to the research. The Act specifies what the independent medical practitioner must take into account when deciding if the medical research is in the best interests of the research candidate. And they must inform the research decision-maker or researcher of their determination and the reasons for it.
For more information refer to the Position Statement: Decisions about medical research.
Medical research and advance health directives
Show moreAn advance health directive is a legal document that a person 18 years of age or older, with full legal capacity can complete. It allows the person to provide or withhold consent for specific health care, medical, surgical or dental treatments or procedures, including life-sustaining measures or palliative care, and participation in medical research.
This document is then used if the person is unable to make a treatment and/or medical research decision at the time it is required due to loss of capacity.
Section 110ZR (4) of the Act requires that a research decision-maker must not consent to medical research on a person if the research is inconsistent with any advance health directive in respect to the person.
Similarly, section 110ZS (2) of the Act requires that a researcher must not conduct medical research on a person if the researcher is aware, or ought reasonably to be aware, the research is inconsistent with any advance health directive in respect to the person.
In very limited circumstances the advance health directive may be considered invalid and the health professional may not follow the directive but instead, must consult the first person listed in the legislation who can make a research decision.
Process for obtaining a research decision
Show moreIf the research candidate is unable to make reasonable judgements about their participation in the medical research, the researcher must determine who has authority as the research decision-maker. The way in which the research decision-maker is identified is outlined below in the “Hierarchy of medical research decision-makers”.
Advance Health Directive
If a person has completed an advance health directive which covers the circumstances and treatment required, health professionals and researchers must follow the treatment and/or research decision in the AHD. An AHD may be in the prescribed form or a common law directive.
Plenary vs Limited
A guardian appointed by the State Administrative Tribunal for an adult with a decision-making disability cannot make a research decision unless the guardianship order gives them the authority to do so. Plenary guardians have this authority but limited guardians require specific authority to make research decisions.
Identifying the Research Decision-maker
The enduring guardian of the person cannot make a research decision unless the person has authorised all functions or provided specific authority to make research decisions. If the guardian or enduring guardian does not have authority to make research decisions, then the researcher must determine the research decision-maker by considering the ‘hierarchy of medical research decision-makers’ – see below.
The hierarchy of medical research decision-makers
Show more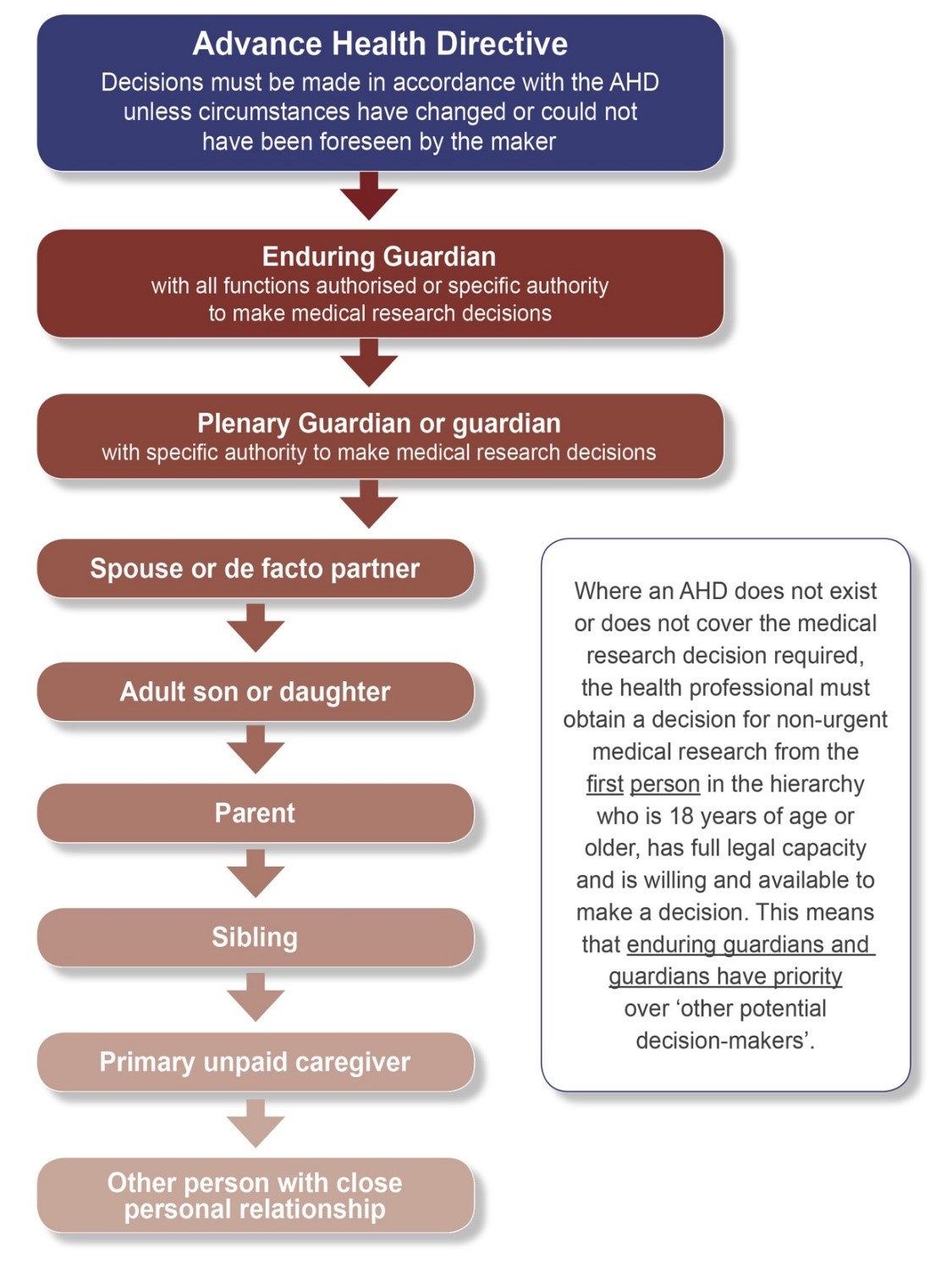
When obtaining a research decision, the researcher must go to the first person in the hierarchy, who is 18 years of age or older, has full legal capacity, and is reasonably available and is willing to make the decision.
If any of these conditions are not met, for example if the potential research decision-maker does not have capacity or is not reasonably available, the health professional can go to the next person in the hierarchy.
Service providers such as allied health professionals and paid support workers have no authority under the Act to make a research decision and are encouraged to provide the treating health professional or researcher with the name and contact details of any legally appointed substitute decision-maker.
It does not matter whether the spouses or de facto partners are different sexes or the same sex; or if either of the persons is legally married to someone else or in another de facto relationship. The Acts Amendment (Lesbian and Gay Law Reform) Act 2002.
A researcher does not have to seek a research decision from the eldest person within any category as there is no distinction in relation to age, therefore all adult children of a person have equal priority.
A person is to be regarded as maintaining a ‘close personal relationship’ with the person needing the research decision if the relationship is maintained through frequent personal contact and a personal interest in the welfare of the person.
The responsibility for making sure that a person being provided with medical research understands the nature and consequences of the research treatment proposed, and for obtaining a research decision from the correct person, lies with the researcher.
If the researcher does not believe the patient has the capacity to make the research decision then it is their responsibility to seek the research decision from the appropriate person.
Urgent medical research without consent
Show moreSection 110ZS of the Act enables a researcher, in certain limited circumstances, to conduct medical research in relation to a research candidate who needs urgent treatment as defined in Section 110ZH to save the person’s life, prevent serious damage to the person’s health or to prevent the person from suffering significant pain or distress. This research must have been approved by a Human Research Ethics Committee.
Certain criteria must be met in order for the medical research to be conducted in the absence of a decision by a research decision-maker.
For more information refer to the Position Statement: Decisions about medical research.
Sterilisation and electroconvulsive therapy are prohibited Section 110ZT of the Act prohibits a research decision-maker from consenting to a procedure for the sterilisation of the research candidate or for electroconvulsive therapy to be performed on the research candidate. Penalties apply for a breach of this provision. The ‘procedure for sterilisation’ takes the meaning given to it under Division 3 of Part 5 of the Act.
When to apply for a guardianship order
Show moreIt is the view of the Public Advocate that an application for a guardianship order should be made to the State Administrative Tribunal when:
- there is conflict about the adult’s capacity to make a decision in relation to the proposed medical research, so the treating health professional requires clarification about capacity
- there is conflict between interested parties about who should be making a research decision
- there is no enduring guardian appointed and there is no one within the description of persons listed in sections 110ZP and 110ZQ to make a research decision
- the person authorised in the Act to make a research decision is unwilling or unable to perform this role or cannot be contacted in a reasonable timeframe
- the person for whom the medical research is proposed objects to the medical research
- notwithstanding the priority list in the hierarchy of research decision-makers, there are disagreements about what medical research will be in the best interests of the person.


